Identifying Spasticity Treatment Gaps in Long-term Care and Community Settings from Ontario Real-world Evidence
Omar D. Khan, MD, FRCPCa; Cathy Vo, PharmDb; Huijuan Yangc, Shoghag Khoudigian-Sinanic; Bradley Millson, MBSd; Riccardo Pedersini, PhDe; Galit Kleiner, MD, FRCPCf
ABSTRACT
Objectives: Post-stroke spasticity (PSS) is a debilitating condition that may be undertreated in patients in long-term care (LTC) facilities. This study evaluated gaps in care for PSS in LTC and community settings in Ontario, Canada. Design: Retrospective, observational, real-world study. Setting and Participants: Patients in Canadian LTC and community settings with ≥1 claim for any botulinum toxin A (BoNT/A) treatment for focal spasticity between 1/1/2010 and 12/31/2019 were included. Methods: Data from the longitudinal IQVIA Ontario Drug Benefit (ODB) claims database were used. Patients were stratified into LTC and community cohorts. Outcomes included total patients treated and total claims per year, treatment rate, mean number of injections per patient per year (two-sided Wilcoxon rank sum test), and time to treatment. Results: Over 10 years, the numbers of patients treated with BoNT/A (2010: n=522; 2019: n=4056), BoNT/A claims for PSS (2010: n=997; 2019: n=12,579), and proportions of claims from LTC residents (2010: 46%; 2019: 52%) increased. The estimated rate of BoNT/A treatment in LTC patients with PSS increased from 33.1% (2015) to 62.5% in 2019, leaving 37.5% of eligible LTC residents untreated. Mean number of injections per patient per year increased from 1.9 in 2010 to ~3.0 in 2017 to 2019 in both LTC and community cohorts. After LTC admission, median time to first physical medicine and rehabilitation physician or neurologist claim was 2.8 years and time to first BoNT/A injection was 2.9 years. Conclusions and Implications: This claims database analysis of patients treated for post-stroke focal spasticity in Ontario revealed a large, undertreated population not receiving BoNT/A therapy and a long time lag between LTC admission and treatment with BoNT/A. These findings highlight the need to increase access to PSS treatment to help reduce patient and caregiver burden.
Authors’ Credentials and Affiliations: aHotel Dieu Shaver Health and Rehabilitation Centre, St. Catharines, ON, Canada; bAllergan, an AbbVie Company, Toronto, ON, Canada; cIQVIA Solutions Canada Inc., Mississauga, ON, Canada; dIQVIA Solutions Canada Inc., Kanata, ON, Canada; eNoesis Healthcare Technologies, Redwood City, CA, USA; fBaycrest Movement Disorders Clinic, Baycrest Health Sciences, ON, Canada
Corresponding Author: Omar Khan, MD, FRCPC, Physician-Physiatrist, ODK Physical Medicine, Hotel Dieu Shaver – Medical Clinic South, St. Catharines, ON L2T 4C2, Canada, Phone: 905-685-1381 ext. 85201, E-mail: [email protected]
Acknowledgments: Allergan (now AbbVie) funded this study and contributed to the study design, the collection, analysis, and interpretation of data, and the review and approval of the final version for publication. All authors had access to relevant data and participated in the review and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided to the authors by Illyce Nunez, PhD, and Marci Mikesell, PhD, of Peloton Advantage, LCC, an OPEN Health company, and was funded by AbbVie.
Funding: This study was supported by Allergan (prior to its acquisition by AbbVie).
Conflicts of Interest: Omar Khan has received honoraria for participation in advisory boards and provision of education and training pertaining to neurotoxin use in spasticity and focal dystonia. He has received sample Botulinum Toxin A from AbbVie, Ipsen, and Merz for clinical patient management. Cathy Vo is an employee of AbbVie and may hold AbbVie stock. Riccardo Pedersini is a health economics consultant for AbbVie. Galit Kleiner, in partnership with Baycrest Health Sciences, owns a use patent for the indication of Botulinum Toxin for paratonia. She has received payments for patent maintenance fees from AbbVie (February 2021, April 2022) while considering licensing of patent (declined). No current relationship. She has received consulting fees and honoraria from AbbVie and Ipsen. She has received sample Botulinum Toxin A from AbbVie, Ipsen, and Merz. Huijuan Yang, Shoghag Khoudigian-Sinani, and Bradley Millson are employees of IQVIA Canada. IQVIA was paid by AbbVie to conduct this research.
Statement of Authorship: Study concept and design: Omar Khan, Bradley Millson, Huijuan Yang, Shoghag Khoudigian-Sinani, Cathy Vo, Galit Kleiner. Acquisition of data: Bradley Millson. Analysis and interpretation of data: Omar Khan, Bradley Millson, Huijuan Yang, Shoghag Khoudigian-Sinani, Riccardo Pedersini, Cathy Vo, Galit Kleiner. Drafting of the manuscript: Omar Khan, Bradley Millson, Huijuan Yang, Shoghag Khoudigian-Sinani, Cathy Vo, Galit Kleiner. Critical revision of the manuscript for important intellectual content: Omar Khan, Cathy Vo, Huijuan Yang, Shoghag Khoudigian-Sinani, Bradley Millson, Riccardo Pedersini, Galit Kleiner.
Data Sharing Statement: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home.”
Status: Peer reviewed.
Submitted: 26 AUG 2024 | Published: 30 SEP 2024
Citation: Khan, Omar D; and Cathy Vo, Huijuan Yang, Shoghag Khoudigian-Sinanic, Bradley Millson, Riccardo Pedersini, Galit Kleiner. Identifying Spasticity Treatment Gaps in Long-term Care and Community Settings from Ontario Real-world Evidence. Canadian Health Policy, SEP 2024. https://doi.org/10.54194/EZWT5430. canadianhealthpolicy.com.
INTRODUCTION
Post-stroke spasticity (PSS) is a motor disorder characterized by involuntary, velocity-dependent increase in tonic stretch reflexes in skeletal muscles with exaggerated tendon jerks, which occur due to an upper motor neuron lesion in the brain or spinal cord.1,2 Without adequate treatment, spasticity may result in reduced quality of life, increased pain, and joint contractures.3 The onset of PSS has often been cited to occur in the first 6-12 months following a stroke.4-8 However, recent literature suggests that PSS can be identified in many patients in as early as 4-6 weeks post-stroke.5,9,10 In a large (N=861) observational study, half of the patients developed PSS during the first month, 25% after 2 months, 12% between 2 and 3 months, and 13% after 3 months following a stroke.11
PSS can negatively affect post-stroke outcomes and quality of life.2,12 Longer rehabilitation clinic stays have been reported in patients with PSS compared with those who do not develop spasticity.4 Complications such as pain, contractures, immobilization in abnormal body positions, and pressure sores can also complicate stroke recovery.2,5,13 These findings highlight the importance of identifying PSS early.
Such issues are a particular concern for patients in long-term care (LTC).14-16 Patients with PSS have greater dependency for activities of daily living than LTC facility (LTCF) residents without spasticity,16 increasing the burden on LTCF staffing and healthcare resources.17,18 It has been suggested that practitioners without specific movement disorder training may not identify and document spasticity adequately, which may hinder follow-up and treatment.16 Furthermore, PSS may be underrecognized and undertreated in the LTC setting. In one study of 209 LTCF residents, only 11% of those determined by a neurologist to have spasticity had an existing spasticity diagnosis and were receiving treatment.16 These findings suggest that post-stroke follow-up and care may be insufficient in LTCFs,19 and stroke patients in general may have limited access to specialist services for spasticity management.13 These barriers to the recognition and treatment of PSS may lead patients and physicians to erroneously consider PSS to be inevitable after a stroke.16 Indeed, LTCF residents living with spasticity often lack awareness of available treatments other than physical and occupational therapy.20
A botulinum toxin type A (BoNT/A) therapy (onabotulinumtoxinA; BOTOX, Allergan, plc; Dublin, Ireland) was first approved in Canada in 1999 for the treatment of spasticity.21-23 Studies have shown improvements in disability outcomes such as pain and caregiver burden with BoNT/A treatment for PSS.24-28
Early treatment with BoNT/A has the potential to improve outcomes for patients with PSS.24 A meta-analysis of trials of early intervention with BoNT/A (<3 months post-stroke) concluded that early treatment may not only improve symptoms (eg, consistently reducing muscle tone in spasticity) but also reduce further development of spasticity, which may support rehabilitation and help prevent complications such as contractures and deformities.24 Also, a longitudinal cohort study (N=83) of PSS patients receiving BoNT/A within 3 months of a stroke reported maximum effects on muscle tone after 1 and 3 months of follow-up.29 These findings show the potential to improve outcomes in PSS with early BoNT/A treatment. Canadian Stroke Best Practice Recommendations support the use of BoNT/A as a first-line therapy to increase range of motion and decrease pain in patients with focal and/or symptomatically distressing spasticity.30
This study aimed to identify and understand the undertreatment and treatment delay in patients with focal spasticity in LTCFs and community settings in Ontario, Canada.
METHODS
Study design
This retrospective, observational, real-world study was conducted using the anonymized, patient-level, longitudinal IQVIA Ontario Drug Benefit (ODB) claims database (FIGURE 1). This database, which contains 100% of all publicly paid drug claims in Ontario, Canada, is actively managed and quality-controlled and captures patient demographic characteristics, specific drugs dispensed, dosage, quantity dispensed, number of days’ supply, service date, pharmacy location, cost, payer, and prescribing physician specialty. De-identified patient IDs are assigned at insurer level, and longitudinal tracking is maintained as long as patients stay within a given plan. As the IQVIA ODB claims database is already in de-identified form, there was no need for ethics approval to conduct the study.
FIGURE 1. Study design.
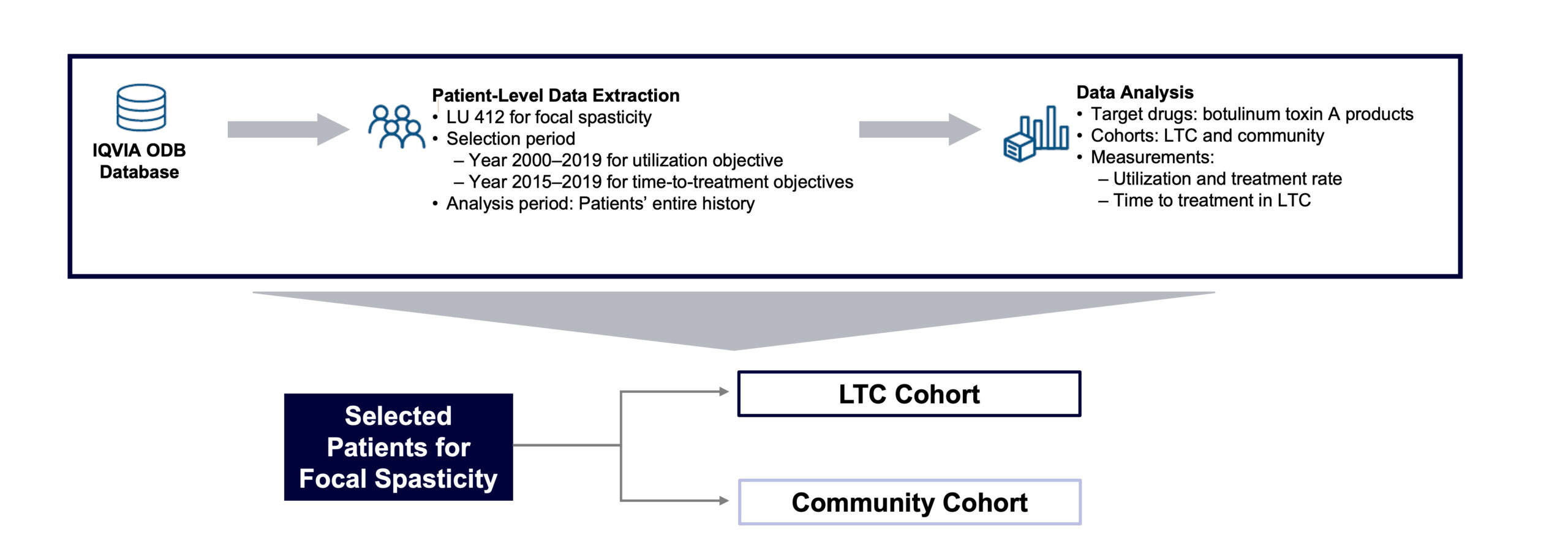
LTC, long-term care; LU, limited use; ODB, Ontario Drug Benefit.
ODB Limited Use (LU) codes indicating if a claim is for specific clinical criteria or conditions for use are included, as well as an LTC flag indicating if a claim is dispensed from an LTC pharmacy. The ODB LU code 412 (for the management of focal spasticity, due to stroke or spinal cord injury in adults) was used to identify claims for post-stroke focal spasticity treatment in this study.
A cross-sectional design was used to report BoNT/A utilization from January 1, 2010, through December 31, 2019, and treatment rate in LTCFs between January 1, 2015, and December 31, 2019. A longitudinal design was used to report time to treatment and other outcomes: patients with ≥1 claim for any BoNT/A (onabotulinumtoxinA, abobotulinumtoxinA [Dysport; Ipsen Biopharmaceuticals, Cambridge, MA, USA], or incobotulinumtoxinA [Xeomin; Merz Pharmaceuticals, Frankfurt, Germany]) for focal spasticity (based on LU code) filed between January 1, 2015, and December 31, 2019, were selected with patients’ full history observed retrospectively. Patients were further stratified into 2 cohorts: LTC and community (ie, with or without LTC flag).
In Canada, onabotulinumtoxinA is indicated for the management of focal spasticity, including upper limb spasticity associated with stroke, and for the symptomatic treatment of lower limb spasticity associated with stroke in adults; abobotulinumtoxinA is indicated for the symptomatic treatment of focal spasticity affecting the upper and lower limbs in patients 2 years of age and older; and incobotulinumtoxinA is indicated for the treatment of spasticity of the upper limb in adults.31-33
Study outcomes
Outcomes included the total number of unique patients per year and total number of claims per year for all BoNT/A treatments in LTC and community cohorts, spasticity treatment rate of all BoNT/A treatments in LTCFs per year over the selection period, mean number of injections per patient per year for individual BoNT/A for focal spasticity in LTC and community cohorts, the proportion of claims prescribed by specialists over the analysis period for all BoNT/A treatments in LTC and community cohorts, mean number of days from LTCF admission to first physical medicine and rehabilitation (PM&R) physician or neurologist claim, and mean number of days from LTCF admission to the first BoNT/A claim for focal spasticity.
Statistical analysis
Descriptive statistics were reported as mean, standard deviation (SD), median, and interquartile range (IQR) for the number of injections per patient per year for all botulinum toxins, as well as for individual botulinum toxins for focal spasticity in the LTC and community cohorts. The two-sided Wilcoxon rank sum test was used to compare the number of injections per patient per year between the LTC and community cohorts and the P value was reported for all botulinum toxins, as well as for individual botulinum toxins in each analysis year. Data management and data analysis were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). The estimated treatment rate of LTCF residents with focal spasticity who received BoNT/A treatment in Ontario was calculated using published estimates of LTCF residents with stroke in Ontario and rates of severe post-stroke focal spasticity, combined with the numbers of LTCF patients treated with BoNT/A for focal spasticity identified in the current analysis.4,34-37 The treatment rate was calculated by dividing LTCF patients receiving BoNT/A claims for focal spasticity (LU code 412) by the estimated total LTC residents with severe post-stroke focal spasticity in Ontario.
RESULTS
BoNT/A utilization and treatment rate for focal spasticity in LTC
Over a 10-year period, the total number of unique patients receiving BoNT/A for spasticity increased more than 7-fold (from 522 in 2010 to 4056 in 2019; see FIGURE 2), the total number of BoNT/A claims for focal spasticity steadily increased (from 997 in 2010 to 12,579 in 2019), and proportions of claims from LTC residents (2010: 46%; 2019: 52%) also increased. The proportion of patients receiving BoNT/A for focal spasticity who were residing in an LTCF increased from 43% in 2010 to 52% in 2019. The estimated rate of BoNT/A treatment for LTCF residents with focal spasticity increased substantially over the 5-year selection period, from 33.1% in 2015 to 62.5% in 2019 (FIGURE 3). However, in 2019, there remained an estimated 37.5% of LTCF residents with focal spasticity who were not receiving BoNT/A therapy.
Among patients receiving BoNT/A injections for focal spasticity in this database, the mean number of injections per patient per year in the LTC and community cohorts increased from 1.9 per year in 2010 to 3.0 per year in 2017 and remained steady at approximately 3 injections per patient per year up to 2019. Results were generally comparable between the LTC and community cohorts, except for 2010 and 2017, when the average number of injections was higher in the LTC cohort compared with the community cohort (P <.05) (FIGURE 4). The mean number of BoNT/A injections per patient is presented in TABLE 1. Overall, the majority of BoNT/A claims were prescribed by PM&R physicians (LTC: 65.6%; community: 82.2%), although the proportion of BoNT/A claims prescribed by neurologists was greater for the LTC (27.2%) than the community cohort (11.3%; FIGURE 5).
FIGURE 2. Number of patients receiving botulinum toxin type A for focal spasticity by year (2010–2019) in LTC and community cohorts of the ODB claims database.
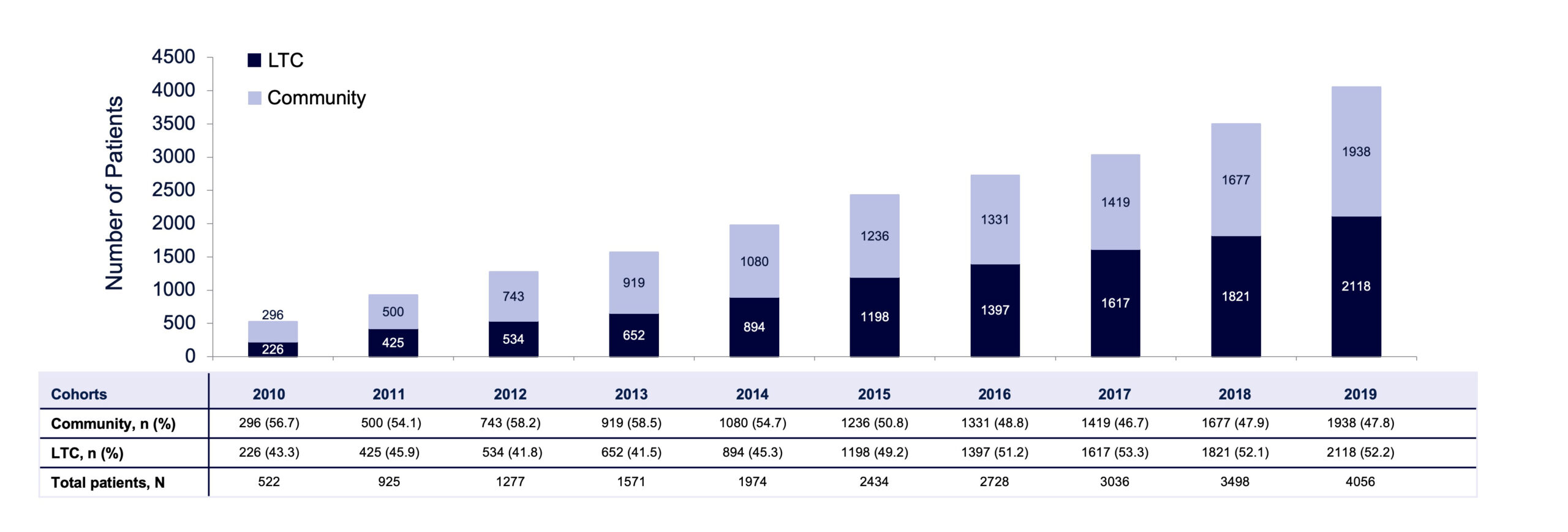 LTC, long-term care; ODB, Ontario Drug Benefit.
LTC, long-term care; ODB, Ontario Drug Benefit.
FIGURE 3. Estimated rate of BoNT/A treatment for post-stroke focal spasticity in LTC in Ontario by year (2015–2019).
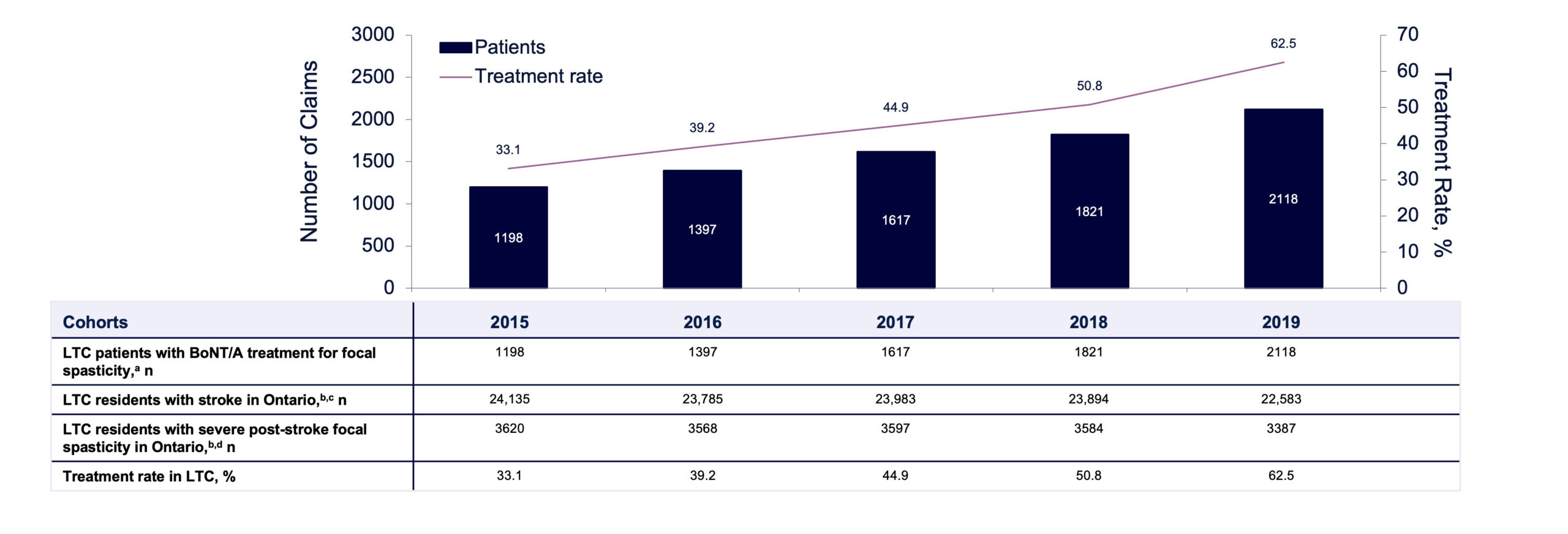 BoNT/A, botulinum toxin type A; LTC, long-term care. aAn assumption was made that all LTC patients with botulinum toxin were for post-stroke focal spasticity treatment. bBased on data from the Canadian Institute for Health Information (CIHI). Profile of Residents in Residential and Hospital-Based Continuing Care, Quick Stats (2016–2019).34-37 cThe percentage of stroke patients in 2015 is based on statistics from 2016, as the 2015 CIHI report was not available. dBased on data from Urban PP, et al. Stroke. 2010;41(9):2016-2020.4
BoNT/A, botulinum toxin type A; LTC, long-term care. aAn assumption was made that all LTC patients with botulinum toxin were for post-stroke focal spasticity treatment. bBased on data from the Canadian Institute for Health Information (CIHI). Profile of Residents in Residential and Hospital-Based Continuing Care, Quick Stats (2016–2019).34-37 cThe percentage of stroke patients in 2015 is based on statistics from 2016, as the 2015 CIHI report was not available. dBased on data from Urban PP, et al. Stroke. 2010;41(9):2016-2020.4
FIGURE 4. Mean number of BoNT/A injections per patient for focal spasticity by year (2010–2019), LTC and community cohorts of the ODB claims database. One injection was defined as one single claim.
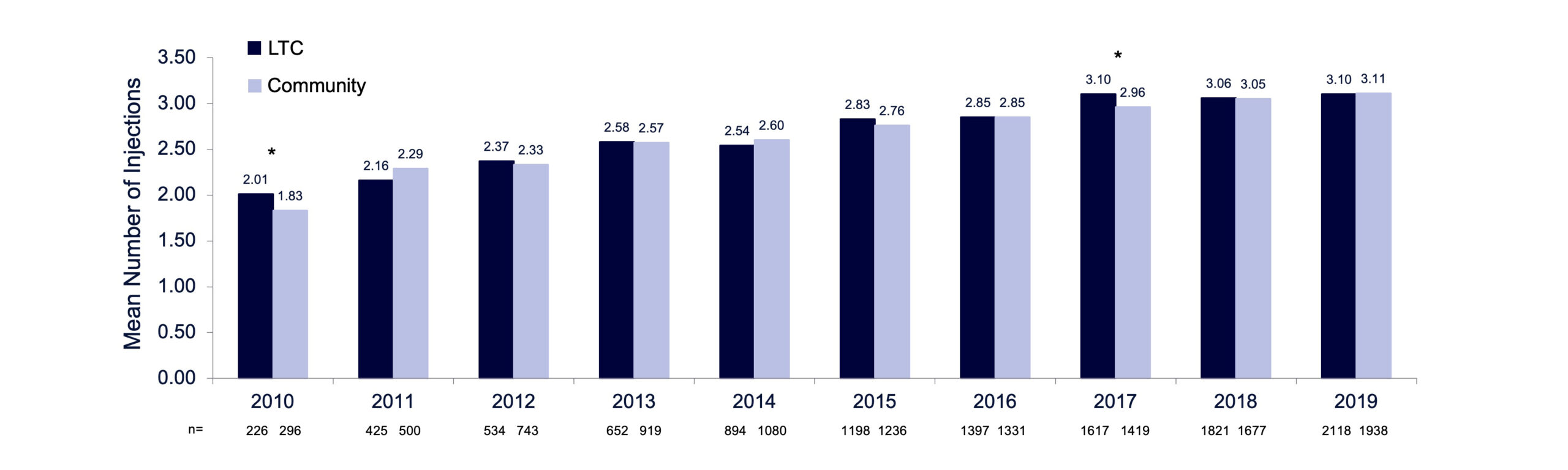
LTC, long-term care; ODB, Ontario Drug Benefit. *P<0.05; LTC vs community cohort, two-sided Wilcoxon rank sum test.
TABLE 1. Mean number of BoNT/A injections per patient for focal spasticity each year within the analysis period, in the total population.
| Analysis Year |
Community Cohort | LTC Cohort | P Valuea | ||||
| Number of Patients | Number of Injections per Patient | Number of Patients | Number of Injections per Patient | ||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| 2010 | 296 | 1.83 (0.99) | 1 (1, 3) | 226 | 2.01 (1.03) | 2 (1, 3) | .030 |
| 2011 | 500 | 2.29 (1.14) | 2 (1, 3) | 425 | 2.16 (1.11) | 2 (1, 3) | .07 |
| 2012 | 743 | 2.33 (1.21) | 2 (1, 3) | 534 | 2.37 (1.21) | 2 (1, 3) | .61 |
| 2013 | 919 | 2.57 (1.31) | 2 (1, 4) | 652 | 2.58 (1.40) | 2 (1, 4) | .93 |
| 2014 | 1080 | 2.60 (1.27) | 3 (1, 4) | 894 | 2.54 (1.36) | 2 (1, 4) | .19 |
| 2015 | 1236 | 2.76 (1.43) | 3 (2, 4) | 1198 | 2.83 (1.61) | 3 (1, 4) | .64 |
| 2016 | 1331 | 2.85 (1.52) | 3 (2, 4) | 1397 | 2.85 (1.52) | 3 (2, 4) | .84 |
| 2017 | 1419 | 2.96 (1.61) | 3 (2, 4) | 1617 | 3.10 (1.66) | 3 (2, 4) | .008 |
| 2018 | 1677 | 3.05 (1.65) | 3 (2, 4) | 1821 | 3.06 (1.77) | 3 (2, 4) | .88 |
| 2019 | 1938 | 3.11 (1.72) | 3 (2, 4) | 2118 | 3.10 (1.62) | 3 (2, 4) | .29 |
BoNT/A, botulinum toxin type A; IQR, interquartile range; LTC, long-term care; SD, standard deviation. aP values in bold indicate a significant difference at 0.05 level in the distribution of injections between community and LTC cohorts.
FIGURE 5. Proportion of BoNT/A claims for focal spasticity within the analysis period (2010–2019) by physician specialty.
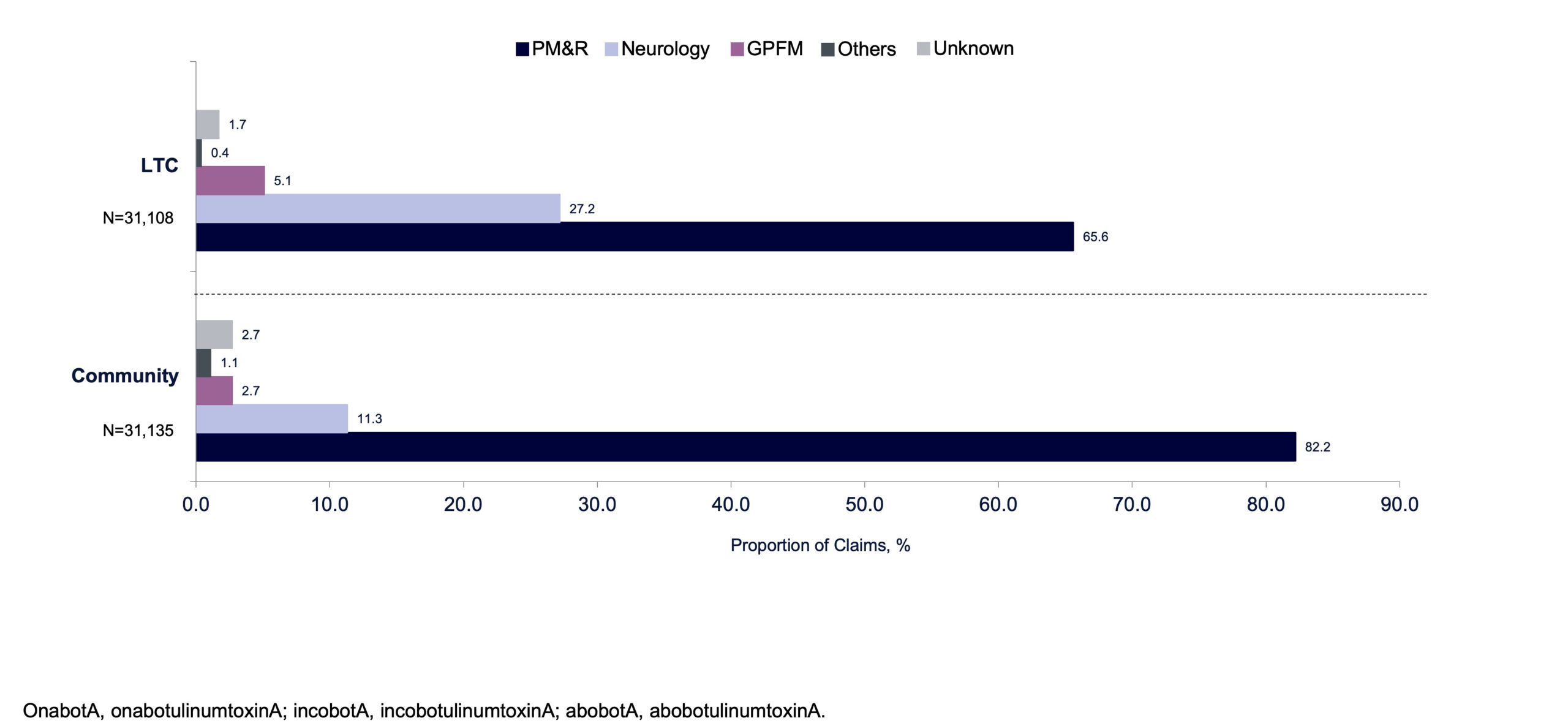 BoNT/A, botulinum toxin type A; GPFM, general practitioner and family medicine; LTC, long-term care; PM&R, physical medicine and rehabilitation.
BoNT/A, botulinum toxin type A; GPFM, general practitioner and family medicine; LTC, long-term care; PM&R, physical medicine and rehabilitation.
Time to treatment with BoNT/A for focal spasticity in LTC
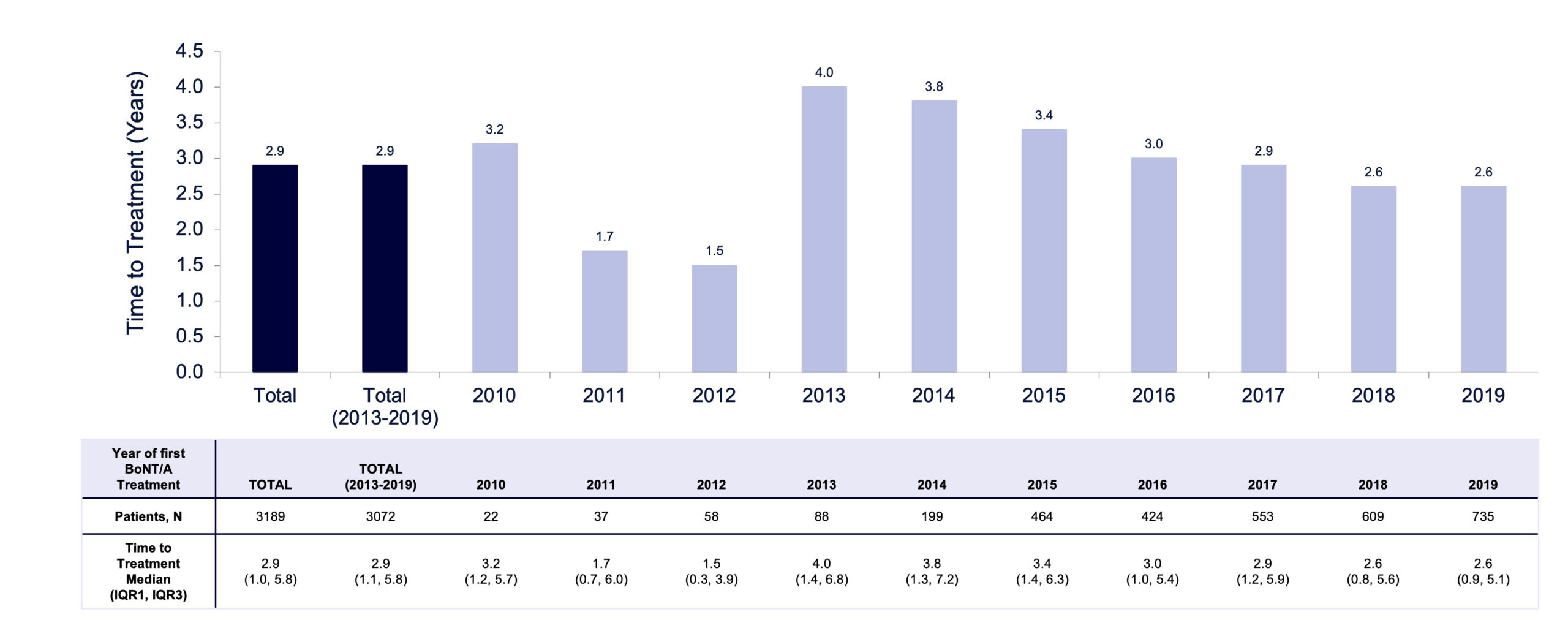
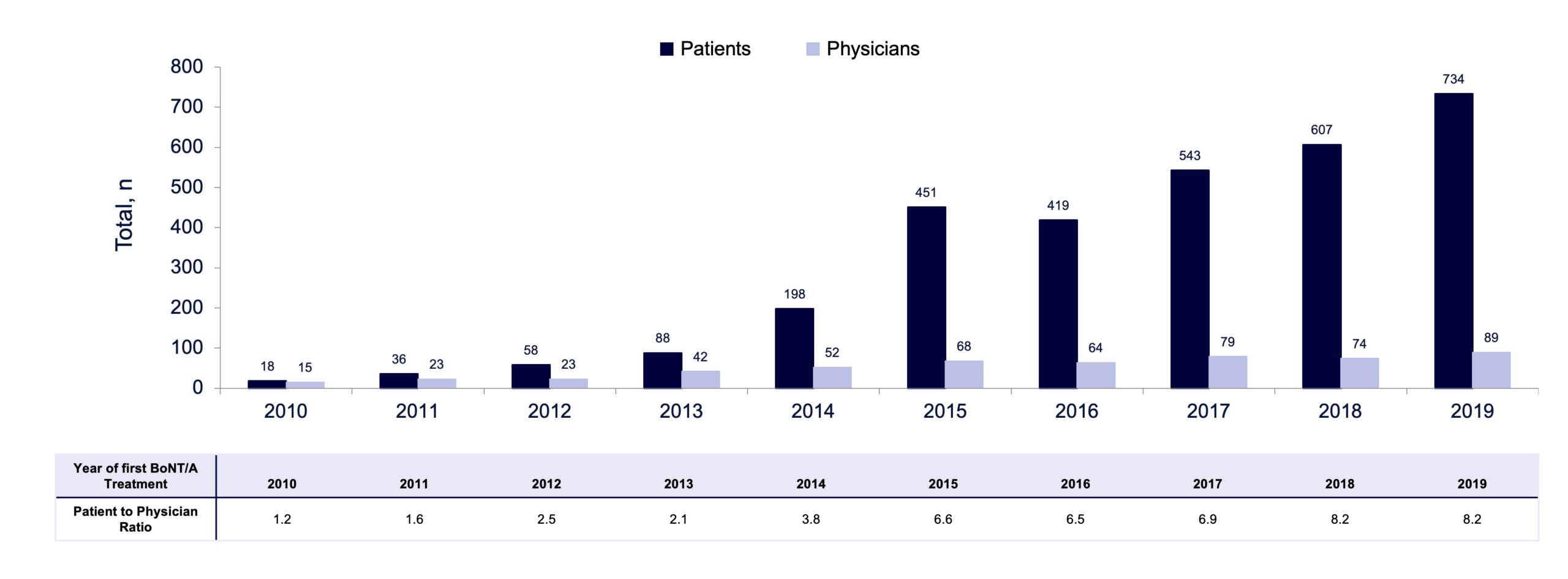
Following LTCF admission, the median time to receiving first BoNT/A injection for focal spasticity was 2.9 years (IQR, 1.0–5.8) between 2010 and 2019 overall (FIGURE 6), with some variability from year to year and a notable increase between 2012 and 2013 as shown in FIGURE 7. Median time to receiving first BoNT/A injection decreased from 4.0 years in 2013 to 2.6 years in 2019. From 2010 to 2019, not only did the volume of patients receiving BoNT/A increase substantially from 18 to 734, but the number of prescribing physicians also increased from 15 to 89 (FIGURE 8). This increase in patients and physicians was most marked in 2013. The ratio of patients to physicians ranged from 1.2 to 2.1 between 2010 and 2013, and increased thereafter up to 8.2 in 2019 (FIGURE 8). The median time from LTCF admission to the first claim prescribed by a PM&R physician or neurologist was 2.8 years (IQR 1.0–5.6).
DISCUSSION
The common occurrence of spasticity in post-stroke patients continues to create substantial public health and economic burden despite the availability of effective treatment, such as BoNT/A.28 This study highlights the gap in care for patients with PSS in LTCFs and provides an understanding of potential barriers to such care. Our observations support the need for future policy considerations to prioritize uniform access to spasticity standards of care for LTCF residents, including increased education and training of LTCF staff on spasticity, earlier specialist referral, and improved logistical coordination.
In this analysis of the IQVIA ODB claims database from 2010 to 2019, the number of BoNT/A claims for PSS increased over time in both LTC and community cohorts. However, the findings indicate that there remains a large, undertreated population of patients with focal spasticity (estimated to be 37.5%) in the LTC setting in Ontario. This study also identified a considerable lag time between LTCF admission and first claim from a PM&R physician or neurologist (2.8 years) or first BoNT/A injection for focal spasticity (2.9 years). Overall, lack of access to specialists represents a barrier to PSS treatment. Increased training, education, and infrastructure within LTCFs for appropriate patient referral, as well as other initiatives, such as telehealth or services connecting health care professionals with spasticity specialists, may help address gaps in the current model of PSS care.38
FIGURE 6. Median time from LTC admission to first prescription and first injection of BoNT/A for focal spasticity.
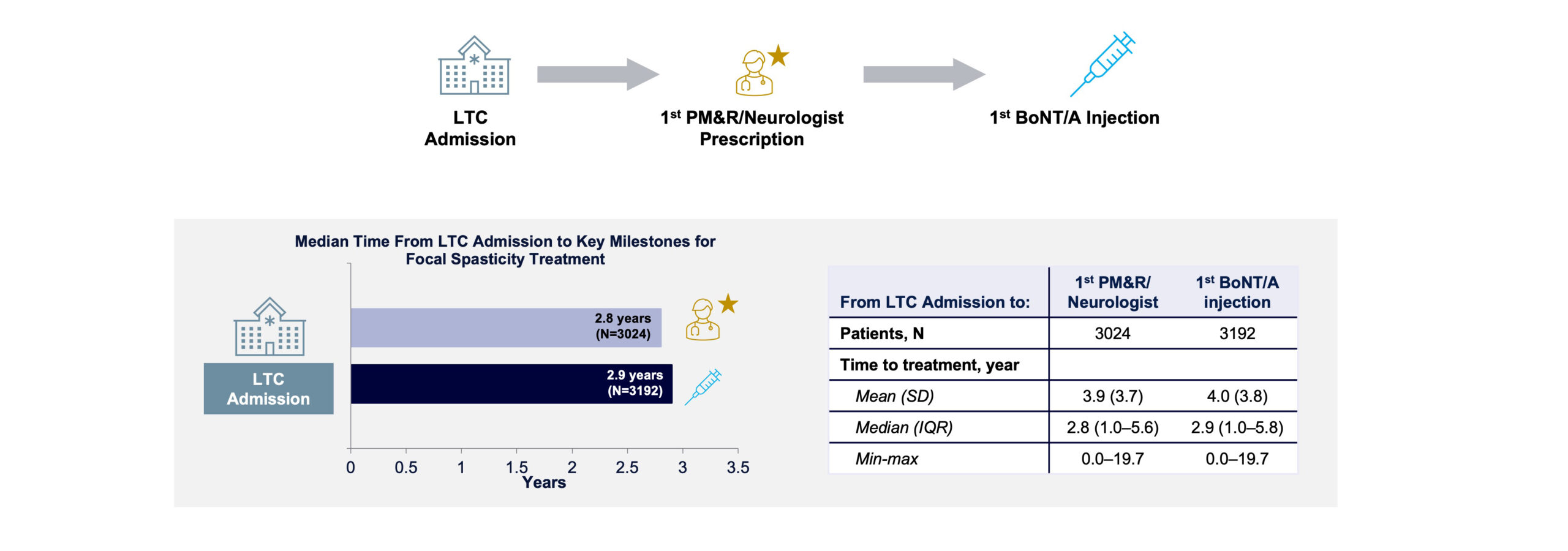 BoNT/A, botulinum toxin type A; IQR, interquartile ratio; LTC, long-term care; max, maximum; min, minimum; PM&R, physical medicine and rehabilitation; SD, standard deviation.
BoNT/A, botulinum toxin type A; IQR, interquartile ratio; LTC, long-term care; max, maximum; min, minimum; PM&R, physical medicine and rehabilitation; SD, standard deviation.
FIGURE 7. Median time from LTC admission to first BoNT/A treatment for focal spasticity treatment in LTCFs.
 BoNT/A, botulinum toxin type A; IQR, interquartile range; LTCFs, long-term care facilities.
BoNT/A, botulinum toxin type A; IQR, interquartile range; LTCFs, long-term care facilities.
FIGURE 8. Total number of patients and prescribing physicians by year of first BoNT/A claim.
 Patients with unknown initiating physician were excluded from this analysis. BoNT/A, botulinum toxin type A.
Patients with unknown initiating physician were excluded from this analysis. BoNT/A, botulinum toxin type A.
A marked increase in time to treatment with BoNT/A was observed between 2012 (1.5 years) and 2013 (4.0 years), in parallel with a sharp increase in the number of prescribing physicians and patients in LTCFs receiving BoNT/A. It is possible that some patients who remained undiagnosed in previous years received treatment with BoNT/A for the first time in 2013 as the availability of BoNT/A treatment services expanded to a broader cohort of LTCFs in Ontario. Although the ratio of patients to physicians increased from 1.2 in 2010 to 8.2 in 2019 (FIGURE 8), this ratio and time to treatment plateaued in 2018 and 2019. This plateau may represent a signal that the Ontario health system was approaching capacity and/or required additional supports in place to expand services and access to specialists. Other factors, such as proximity to an injecting physician or parameters intrinsic to the patient demographics of those LTCFs, could also have affected time to treatment.
Treatment with BoNT/A has been shown to benefit patients with PSS and can limit the potential adverse consequences of remaining untreated, such as worsening disability.24-28 Spasticity management may also affect health care outcome metrics (ie, wound care, pressure ulcers, risk of falls, fractures).39-41 For example, in a 2016 study, Lam et al showed the presence of contractures to be a risk factor for minimal trauma fractures in LTCF residents, and proposed that spasticity management and contracture prevention may reduce risk of fractures.39 A 2020 study discussed how the use of BoNT/A injections for treating spasticity improved hand opening, allowing hand wounds to be cleaned, dressed, and splinted, which resulted in ulcer healing, pain reduction, and reduced caregiver burden.40 Also, spasticity has been found to be a predictor of falls in ambulatory post-stroke patients.41
Access to care for the management of outpatient focal spasticity, including BoNT/A injections, has been shown to vary widely across Canada.42 The current findings indicate reduced or delayed access to BoNT/A treatment in LTCFs in Ontario, which may negatively affect clinical outcomes for patients with PSS. Additional research is warranted to assess gaps in care in other provinces. Current guidelines in Canada specify that all individuals who transition to LTC following a stroke should be assessed by medical, nursing, and rehabilitation professionals as soon as possible after admission and should have access to specialized stroke services.43 In a retrospective cohort study in Canada, stroke was prevalent among LTC patients with high care needs, a population with increased mortality rates.44 The nature of the care gaps evident from the current study underscores the need for a system-wide approach to PSS treatment in LTC settings. For example, evaluation of PSS and BoNT/A treatment should be conducted as quality indicators for LTC of patients with stroke. Addressing other barriers to spasticity care, such as heterogeneity in treatment reimbursement and lack of a specific diagnosis code, may improve spasticity management.45 Together, these efforts may help ensure that patients receive BoNT/A treatment for focal spasticity in a timely manner after LTCF admission.
This study was subject to limitations typical of claims-based analyses. Focal spasticity could have been due to stroke or spinal cord injury, and stroke diagnosis could not be confirmed from the claims data. However, a stroke diagnosis is common in patients admitted to LTCFs (10% to 18%) and usually precedes LTCF admission.46,47 In the authors’ real-world clinical experience, patients receiving BoNT/A injections after a stroke are admitted to LTC specifically because of their disability due to stroke, which is the primary diagnosis. However, the time to treatment for LTC and community patients cannot be compared due to lack of information about the timing of stroke episodes. The time of entry into LTC for patients was inferred by their first LTC claim; it was assumed that the first claim for PM&R physician or neurologist visit46 occurred during the first visit, but patients may have been assessed by a PM&R physician or neurologist before the first prescription claim.
Also, the ODB claims database includes public claims data only, and findings may not be generalizable to the private sector. The number of eligible and treatable patients was estimated from the literature and assumed that all patients with severe PSS were eligible and should be treated; the actual number of eligible and treatable patients may be fewer or greater. Furthermore, the scope of this study was limited to treatment with BoNT/A, and it is possible that “untreated” patients may have been receiving other treatments for spasticity (eg, antispasmodics, physical therapy).
Historically, due to a lack of infrastructure from the Ministry of Health and Long-Term Care (MOHLTC), the management and treatment of patients with PSS in LTC has been, in part, contingent on external support of industry partners to allow for referral pathways and tone assessments to be conducted. However, these programs are disjointed and not widely available. Despite attempts to improve residents’ access to spasticity specialists and enhance treatment, it is now evident this approach alone has not been adequate to provide sufficient or equal service access. Without infrastructure and support, the “mobile clinics” of physicians attending LTCFs and treating at the bedside are difficult to sustain and standardize across all LTCFs in the province. The federal government and the Health Standards Organization (HSO) have committed to developing LTC standards and guidelines to ensure seniors “can access the safe, quality health care they need and deserve.”48 The Ontario MOHLTC is also working toward reforming LTC standards to address the fragmentation and variability of care within Ontario.48 By working together, all levels of government, health care providers, and other stakeholders in the sector can help create a sustainable and effective system of care that improves outcomes for stroke patients and their families.
CONCLUSIONS
In this analysis of claims for BoNT/A injection in the IQVIA ODB database, we observed a significant gap in care for LTCF residents in Ontario, with a large, undertreated volume of patients with spasticity and considerable lag time of 2.9 years to receive treatment from time of admission. This gap in care exists despite the fact that BoNT/A is considered standard of care for this debilitating condition in Canada. Establishing a stroke rehabilitation program that incorporates tone assessment, treatment services, and staff training as part of a standardized protocol across all LTCFs could help address care gaps. Additional health policy research on stroke rehabilitation is needed to enhance models of care in Ontario, to better define referral pathways, and to establish a framework for delivering specialist interdisciplinary care to LTC residents. This study underscores the need for all LTC residents with stroke to have timely and equitable access to the care that they need, with BoNT/A available as a core treatment for PSS.
